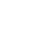Steelmaking begins with ironmaking. Steel comes from pig iron. Pig iron smelted from iron ore has high carbon content and many impurities (such as silicon, manganese, phosphorus, sulfur, etc.). Therefore, pig iron lacks plasticity and toughness, and has poor mechanical properties. It cannot be subjected to pressure processing except for melting and casting, which limits its use.
In order to overcome these shortcomings of pig iron and make it play a greater role in the industry, it is also necessary to use oxygen from various sources at high temperatures to remove the impurities in pig iron to a certain degree to obtain a certain composition and certain The nature of the iron-carbon alloy-steel. This method of removing impurities in pig iron by oxidation at high temperatures is called steelmaking.
The basic principles of steelmaking
Various impurities in pig iron have greater affinity with oxygen to varying degrees under high temperature environments. Therefore, they can be made into liquid, solid or gaseous oxides by oxidation. The liquid and solid oxides react with the furnace lining and the flux added to the furnace at high temperatures, combine to form slag, and are removed from the furnace during slagging. The gas is also taken out of the furnace by CO when the molten steel is boiling.
In the steelmaking furnace, the oxidation of impurities is mainly achieved by the presence of FeO.
2Fe+O2→2FeO
1. Oxidation of silicon
Si has a greater affinity with oxygen, so the oxidation of silicon is very rapid. It has been completely oxidized to form SiO2 in the early stage of smelting:
Si+2FeO→SiO2+2Fe
At the same time SiO2 reacts with FeO to form silicate:
2FeO+SiO2→2FeO·SiO2
This kind of salt is a very important part of the slag. It interacts with CaO to generate stable compounds 2CaO·SiO2 and FeO. The former is firmly in the slag, and the latter becomes a free component in the slag, which increases the content of FeO in the slag. It is more advantageous to promote the oxidation of impurities. The response is as follows:
2FeO·SiO2+2CaO→2CaO·SiO2+2FeO
2. Oxidation of manganese
Manganese is also an element that is easy to oxidize. The MnO produced by it has a higher melting point. MnO does not dissolve in the molten metal, but it forms a compound with SiO2 that floats on the surface of the liquid metal and becomes a part of the slag.
Mn+FeO→MnO+Fe
2MnO+SiO2→2MnO·SiO2
The oxidation reaction of silicon and manganese releases a lot of heat, which can quickly increase the furnace temperature (this is particularly important for converter steelmaking) and greatly accelerate the carbon oxidation process.
3. Oxidation of carbon element
The oxidation of carbon needs to absorb a large amount of heat energy, so it must be carried out at a higher temperature. The oxidation of carbon is a very important reaction in the steelmaking process:
C+FeO→CO+Fe
Because CO gas is generated when carbon is oxidized, it acts as a strong agitation when it escapes from the liquid metal. This effect is called "boiling". The result of boiling can promote the uniformity of the composition and temperature of the molten pool, accelerate the reaction between the metal and the slag interface, and also help to remove the gas and inclusions in the steel.
4. Oxidation of phosphorus element
The oxidation of phosphorus can occur at a temperature that is not too high. The dephosphorization process consists of a combination of several reactions. The reactions are as follows:
2P+5FeO→P2O5+5Fe
P2O5+3FeO→3FeO·P2O5
When there is enough CaO in the alkaline slag, the following reactions will occur:
3FeO·P2O5+4CaO→4CaO·P2O5+3FeO
The 4CaO·P2O5 produced by is a stable compound, which is firmly held in the slag, thus achieving the purpose of dephosphorization.
It must be noted that during the deoxidation process of molten steel, deoxidizers such as ferrosilicon and ferromanganese must be added. Therefore, after deoxidation, the slag is often acidic, and 3FeO·P2O5 is destroyed, and P2O5 is reduced from it, and P2O5 is unstable. Oxide, it is easily reduced by carbon at high temperature, resulting in phosphorus recovery. This also shows that it is very difficult to remove phosphorus in an acid furnace. In order to prevent this phenomenon, it is necessary to appropriately increase slag basicity and slag amount, and improve slag oxidation.
5. Oxidation of sulfur
Sulfur exists in the form of FeS. When there is enough CaO in the slag, the sulfur can also be removed. The reaction is as follows:
FeS+CaO→CaS+FeO
The generated CaS is not soluble in molten steel, but forms slag floating on the surface of molten steel.
The above reaction is a reversible reaction, and it is carried out in the slag containing FeO. When FeO interacts with CaS, the sulfur will return to the molten steel, so the desulfurization efficiency increases as the FeO content in the slag decreases.
When the slag contains enough carbon, the reaction is different:
CaO+FeS+C→CaS+Fe+CO
Because carbon deprives FeO of oxygen, it loses the possibility of CaS interacting with FeO, so that the reaction cannot proceed in the reverse direction. This is why the desulfurization of electric furnace steelmaking is more complete than the other two methods.
In the process of desulfurization, manganese also plays a role in promoting desulfurization. The process is as follows:
FeS+MnO→MnS+FeO
The generated MnS is almost insoluble in molten steel and enters the slag. Therefore, the effect of desulfurization increases with the oxidation of manganese.
6. Deoxygenation of FeO
After the above series of oxidation reactions, although the impurities are oxidized to achieve the purpose of removal, but also due to the oxidation results, the molten steel contains more FeO, that is to say, there is a large amount of oxygen in the molten steel, which will give the steel strip This is a great hazard. On the one hand, the steel has a lot of bubbles; on the other hand, it also causes the steel to appear hot and cold brittle, and the hazard increases with the increase of carbon content.
Therefore, at the end of the steelmaking process, we must also try to remove a large amount of oxygen present in the molten steel. The commonly used method is to add some deoxidizers, such as ferromanganese, ferrosilicon, aluminum, etc., to the molten steel. They strongly extract oxygen from FeO to achieve the purpose of deoxidation. The reaction is as follows:
FeO+Mn→MnO+Fe
2FeO+Si→SiO2+2Fe
3FeO+2Al→Al2O3+3Fe
7. The role of slag
The entire steelmaking process consists of two processes: oxidation and reduction. The oxidation of carbon, silicon, manganese, and phosphorus is usually called the reaction in the oxidation period, and desulfurization and deoxidation are called the reaction in the reduction period. It can be seen from the above reaction formulas that in order to remove impurities in the metal, many factors must be considered, but the most important factor is slagging and slag removal.
Slag has the following important roles in the steelmaking process:
①The slag should ensure that the steelmaking process proceeds in a certain reaction direction (oxidation or reduction).
②The slag should ensure the maximum removal of harmful impurities (phosphorus and sulfur) in the metal, and prevent the gas in the furnace gas (nitrogen and hydrogen) from entering the metal.
③The slag should ensure the minimum loss of iron and other valuable elements during operation.
The basic method of steelmaking
①Converter steelmaking
Converter steelmaking method is a steelmaking method that uses air or oxygen to oxidize the elements in the molten iron to the specified limit by adopting bottom blowing, side blowing and top blowing to obtain steel with qualified composition.
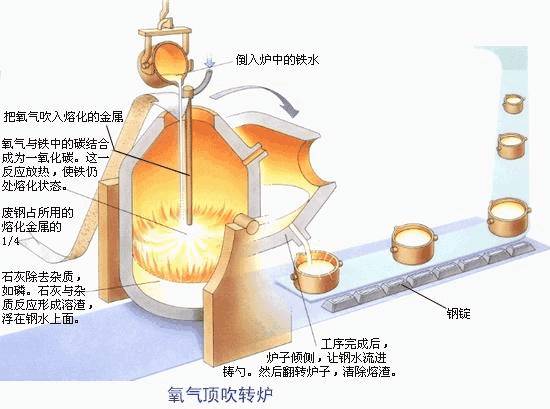
② Electric furnace steelmaking
The electric furnace uses electric energy to transform into heat energy to make steel. There are two commonly used electric furnaces: electric arc furnace and induction electric furnace. Electric arc furnaces are the most widely used and are suitable for smelting high-quality steel and alloy steel; induction furnaces are used for smelting high-grade alloy steel and non-ferrous alloys.
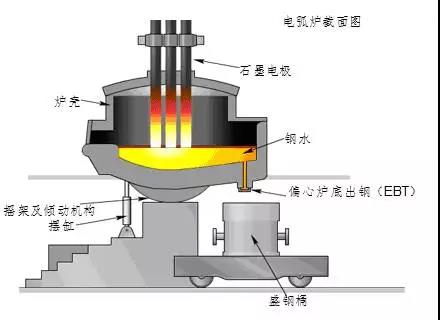
③Open hearth steelmaking
With the development of industry, a large amount of scrap steel has been accumulated in the metal processing industry. At that time, it was not possible to re-blown it into steel with a converter, so steelmakers looked for a steelmaking method using scrap steel as raw material. In 1864, the Frenchman Martin invented the open-hearth steelmaking method.
The rapid development of the oxygen top-blown converter steelmaking method has gradually replaced the open-hearth steelmaking method. With the advancement of science and technology, some new steelmaking methods continue to appear, such as the vacuum treatment of molten steel, electroslag furnace smelting, and vacuum induction electric furnace smelting, which have been used more and more.
Post time: Aug-02-2021